Tantus ensures fast and efficient tableting of dietary supplements. Our specialists assist in developing formulas suitable for producing attractive and durable tablets, with a composition that delivers the desired results. Only high-quality raw materials with the appropriate certifications are used. To ensure the tableting process runs smoothly, we apply strict quality control procedures.

Tablets are one of the most frequently chosen forms of dietary supplements by consumers. Their accessibility and popularity directly impact consumer trust, sales, and product performance. Professional tableting and contract manufacturing provide measurable results, allowing our clients to maximize benefits.
Our company offers tablet production in the following sizes:
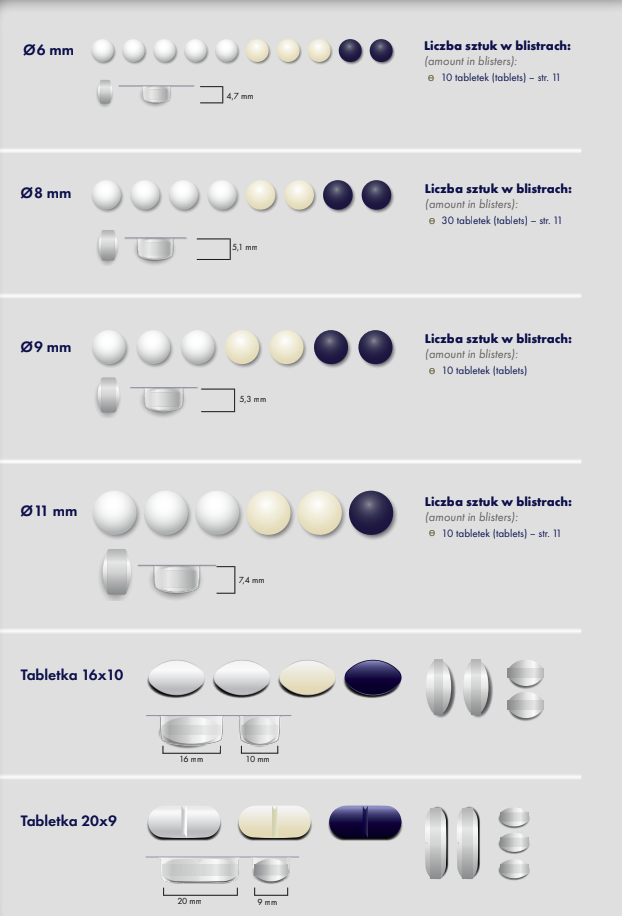
Depending on client needs, we also provide contract manufacturing of tablets in different shapes, sizes, and colors to make the product visually appealing to the end consumer.
Tablet production can be complemented by blister packaging, which enhances convenience, visual appeal, and protection from external factors.
Additionally, tablets can be coated to mask unpleasant tastes and odors of active substances, making them more acceptable and easier to consume. Supplements in tablet form enjoy high acceptance among end users.



At Tantus, contract manufacturing of dietary supplements involves continuous improvement of oral dosage forms to deliver visually appealing products with consumer-friendly properties. The process is planned and refined in every detail. Direct compression always begins with answering key questions:
Already during the formulation phase, factors such as the frequency of intake and the active ingredient’s release profile (immediate, enteric, or delayed release) must be considered.
Proper direct compression must also anticipate and eliminate potential issues. This includes flowability and solubility of the active substance, as well as sensitivity of the tablet’s performance and appearance to moisture and other environmental factors like oxidation, light, and temperature.

